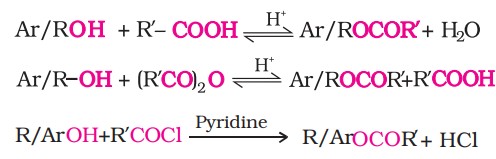
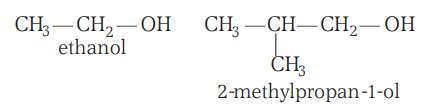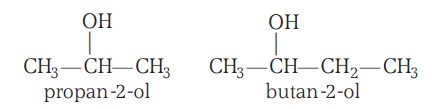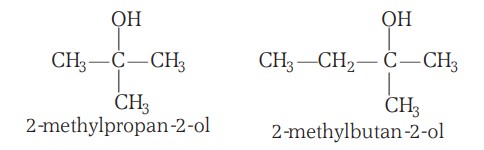Classification Of Alcohols (II)
Based on the Number of -OH Groups Present:
The alcohols may be monohydric (i.e., have one -OH group), dihydric (have two -OH groups), trihydric (have three -OH groups):
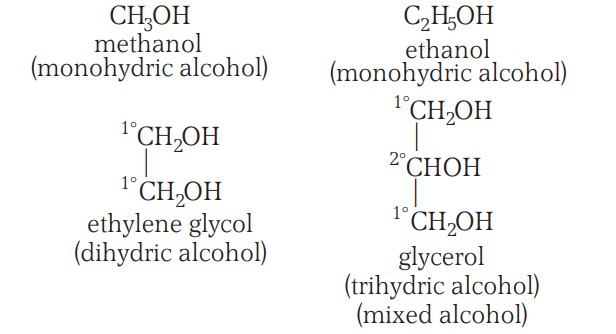


Preparation Of Alcohols (From Alkenes) I
(i) By acid catalysed hydration: Alkenes react with water in the presence of acid as catalyst to form alcohols. In case of unsymmetrical alkenes, the addition reaction takes place in accordance with Markovnikov’s rule.
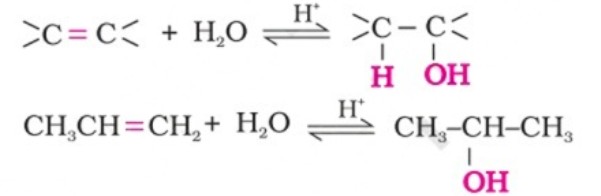
Mechanism - 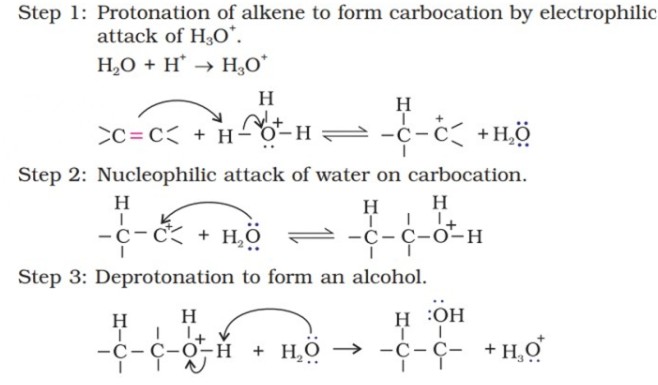


Preparation Of Alcohols (From Alkenes) II
(ii) By hydroboration-oxidation:
Diborane reacts with alkenes to give trialkyl boranes as an addition product. This is oxidised to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide.
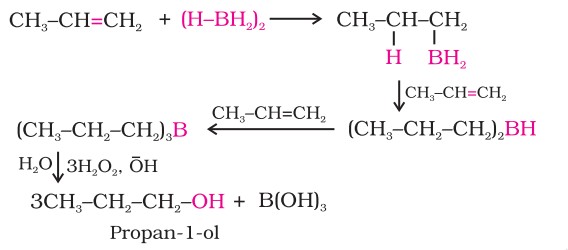
The addition of borane to the double bond takes place in such a manner that the boron atom gets attached to the sp2 carbon carrying a greater number of hydrogen atoms. The product formed seems like the reaction has undergone an anti-markonikoff's reaction and this reaction gives excellent yield.


Preparation Of Alcohols (From Carbonyl Compounds) I
(i) By reduction of aldehydes and ketones: Aldehydes and ketones are reduced to the corresponding alcohols through catalytic hydrogenation.
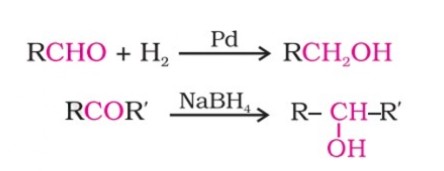
The usual catalyst is a finely divided metal such as platinum, palladium or nickel.
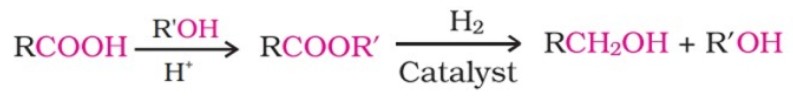
(ii) By reduction of carboxylic acids and esters:
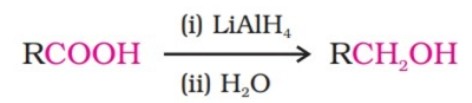
It is also prepared by treating aldehydes and ketones with sodium borohydride or lithium aluminium hydride.
Aldehydes yield primary alcohols whereas ketones give secondary alcohols.


Preparation Of Alcohols (From Grignard Reagents) I
Alcohols are produced by the reaction of Grignard reagents with aldehydes and ketones.
The first step of the reaction is the nucleophilic addition of the Grignard reagent to the carbonyl group to form an adduct.
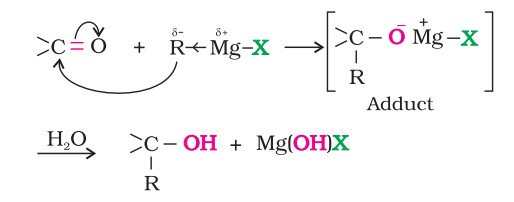
Hydrolysis of the adduct yields an alcohol.


Preparation Of Phenols I
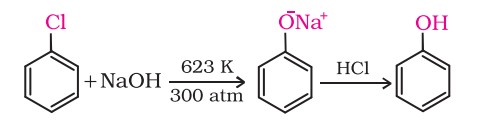
1. From haloarenes
Chlorobenzene is fused with NaOH at 623K and 320 atmospheric pressure. Phenol is obtained by acidification of sodium phenoxide.
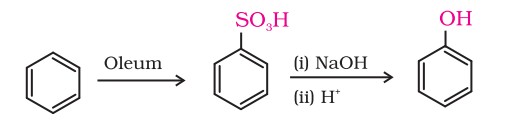
2. From benzenesulphonic acid
Benzene is sulphonated with oleum, and the resulting benzene sulphonic acid is then converted to sodium phenoxide by heating with molten sodium hydroxide. Acidification of the sodium salt gives phenol.


Preparation Of Phenols II
3. From diazonium salts
A diazonium salt is formed by treating an aromatic primary amine with nitrous acid at 273-278 K. Diazonium salts are hydrolysed to phenols by warming with water or by treating with dilute acids.
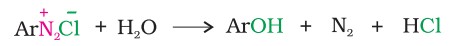
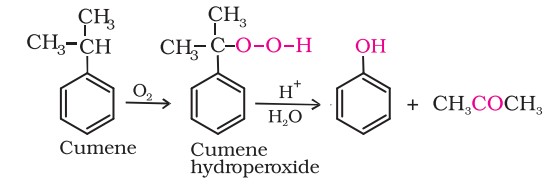
4. From cumene
Phenol is manufactured from the hydrocarbon, cumene. Cumene (isopropylbenzene) is oxidised in the presence of air to cumene hydroperoxide. It is converted to phenol and acetone by treating it with dilute acid.


Physical Properties Of Alcohols
(i) Boiling Points
Alcohols have a higher boiling point than haloalkanes of comparable molecular mass because alcohols have intermolecular hydrogen bonding. As the number of carbon atoms increases, the boiling point increases. The boiling point decreases with an increase in branching in the carbon chain.
(ii) Solubility
Alcohols are soluble in water due to their ability to form hydrogen bonds with water. As the number of carbon atoms increases, solubility decreases.


Chemical Properties Of Alcohols And Phenols (I)
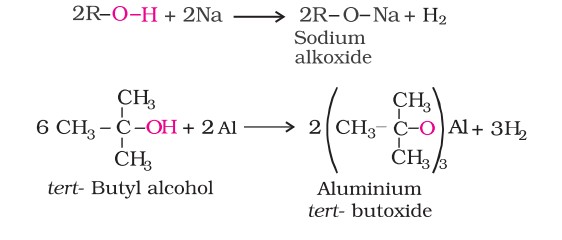
1. Acidity of alcohols and phenols
Alcohols and phenols react with active metals such as sodium, potassium and aluminium to yield corresponding alkoxides/phenoxides and hydrogen. In addition to this, phenols react with aqueous sodium hydroxide to form sodium phenoxides and water


Chemical Properties Of Alcohols And Phenols (III)
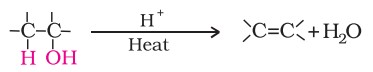
3. Dehydration
Alcohols undergo dehydration (the removal of a molecule of water) to form alkenes when treated with a protic acid, such as concentrated sulfuric acid or phosphoric acid, or with catalysts like anhydrous zinc chloride or alumina.
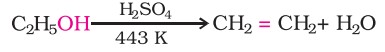
Ethanol undergoes dehydration by heating it with concentrated sulphuric acid at 443 K.
Secondary and tertiary alcohols are dehydrated under milder conditions -
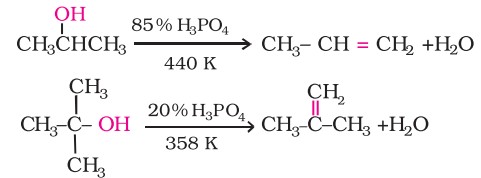
Thus, the relative ease of dehydration of alcohols follows the following order: Tertiary > Secondary > Primary


Chemical Properties Of Alcohols And Phenols (IV)
4. Oxidation
Oxidation of alcohols involves the formation of a carbon-oxygen double bond with cleavage of an O-H and C-H bond. Such a cleavage and formation of bonds occur in oxidation reactions.
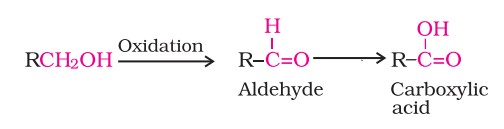
These are also known as dehydrogenation reactions as these involve loss of dihydrogen from an alcohol molecule. Depending on the oxidising agent used, a primary alcohol is oxidised to an aldehyde which in turn is oxidised to a carboxylic acid. Some of these agents include - PCC, CrO3, Cu at 573K, etc.


Chemical Properties Of Alcohols And Phenols (V)
5. Electrophilic aromatic substitution
(i) Nitration: With dilute nitric acid at low temperature (298 K), phenol yields a mixture of ortho and para nitrophenols.
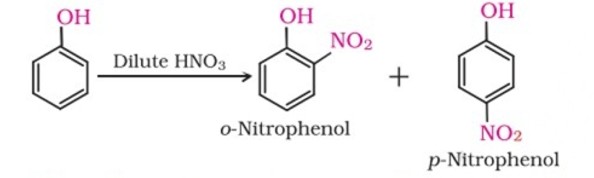
With concentrated nitric acid, phenol is converted to 2,4,6-trinitrophenol. The product is commonly known as picric acid. The yield of the reaction product is poor.



Chemical Properties Of Alcohols And Phenols (VI)
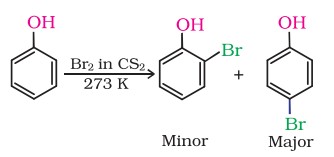
(ii) Halogenation
On treating phenol with bromine, different reaction products are formed under different experimental conditions.
(a) When the reaction is carried out in solvents of low polarity such as CHCl3 or CS2 and at low temperature, monobromophenols are formed.
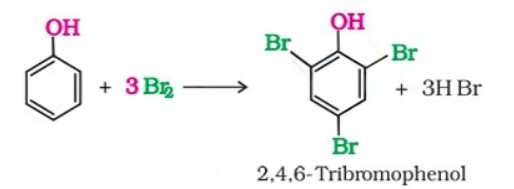
(b) When phenol is treated with bromine water, 2,4,6-tribromophenol is formed as white precipitate.


Chemical Properties Of Alcohols And Phenols (VII)
6. Kolbe’s reaction
Phenoxide ion generated by treating phenol with sodium hydroxide is even more reactive than phenol towards electrophilic aromatic substitution.
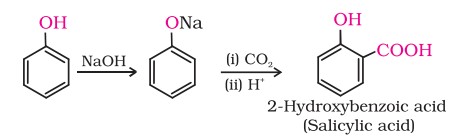
Hence, it undergoes electrophilic substitution with carbon dioxide, a weak electrophile. Ortho-hydroxybenzoic acid is formed as the main reaction product.
7. Oxidation
Oxidation of phenol with chromic acid produces a conjugated diketone known as benzoquinone. In the presence of air, phenols are slowly oxidised to dark coloured mixtures containing quinones.
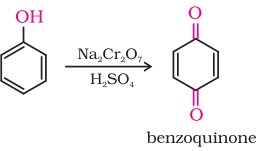


Chemical Properties Of Alcohols And Phenols (VIII)
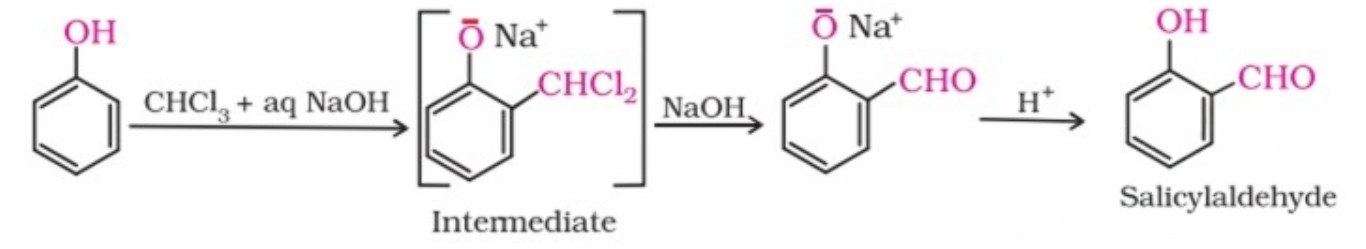
8. Reimer-Tiemann reaction
On treating phenol with chloroform in the presence of sodium hydroxide, a –CHO group is introduced at ortho position of benzene ring. The intermediate substituted benzal chloride is hydrolysed in the presence of alkali to produce salicylaldehyde.
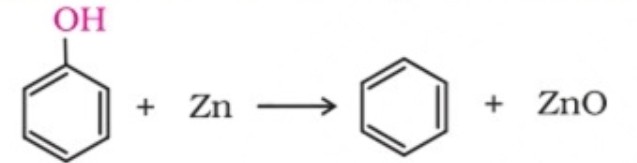
9. Reaction of phenol with zinc dust
Phenol is converted to benzene on heating with zinc dust.


Ethers Introduction
Ethers are classified as simple or symmetrical, if the alkyl or aryl groups attached to the oxygen atom are the same, and mixed or unsymmetrical, if the two groups are different.
Diethyl ether is a symmetrical ether, whereas methoxyethane is an unsymmetrical ethers.


Preparation Of Ethers (I)
1. By dehydration of alcohols
Alcohols undergo dehydration in the presence of protic acids. The formation of the reaction product, alkene or ether, depends on the reaction conditions.
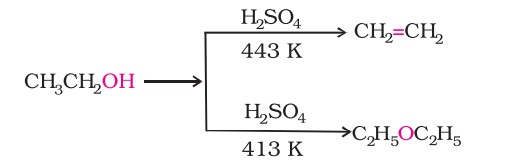
For example, ethanol is dehydrated to ethene in the presence of sulphuric acid at 443K. At 413K, ethoxyethane is the primary product.


Preparation Of Ethers (II)
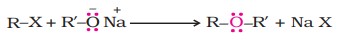
2. Williamson synthesis
It is a crucial laboratory method for the preparation of both symmetrical and unsymmetrical ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide.
Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method. The reaction involves an SN2 attack of an alkoxide ion on a primary alkyl halide.
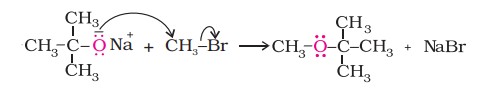


Physical Properties Of Ethers
(i) The C-O bonds in ethers are polar and thus, ethers have a net dipole moment. The weak polarity of ethers does not appreciably affect their boiling points, which are comparable to those of the alkanes of comparable molecular masses but are much lower than the boiling points of alcohols.
The large difference in boiling points of alcohols and ethers is due to the presence of hydrogen bonding in alcohols.
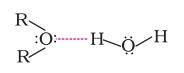
(ii) The miscibility of ethers with water resembles that of alcohols of the same molecular mass. This is because just like alcohols, the oxygen of ether can also form hydrogen bonds with water molecules, as shown:


Chemical Reactions - Cleavage Of C–O Bond In Ethers
The cleavage of the C-O bond in ethers takes place under drastic conditions with an excess of hydrogen halides. The reaction of dialkyl ether gives two alkyl halide molecules.
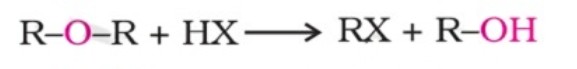
Alkyl-aryl ethers are cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond. The reaction yields phenol and an alkyl halide.
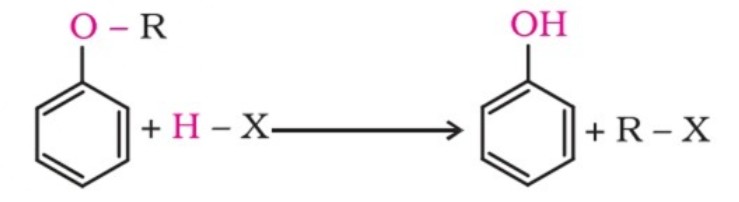
The order of reactivity of hydrogen halides is as follows: HI > HBr > HCl.


Chemical Reactions - Electrophilic Substitution (I)
The alkoxy group (-OR) is ortho-para directing and activates the aromatic ring towards electrophilic substitution in the same way as in phenol.
(i) Halogenation:
Phenylalkyl ethers undergo usual halogenation in the benzene ring, e.g., anisole undergoes bromination with bromine in ethanoic acid, even in the absence of iron (III) bromide catalyst.
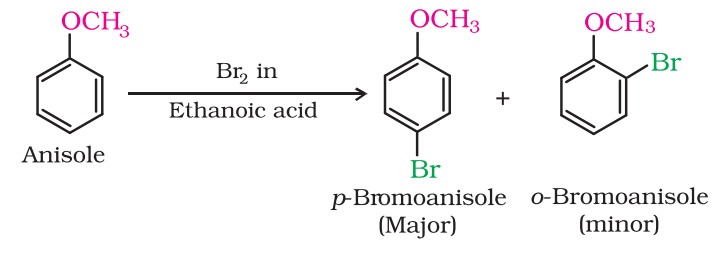
It is due to the activation of the benzene ring by the methoxy group. Para isomer is obtained in 90% yield.


Chemical Reactions - Electrophilic Substitution (II)
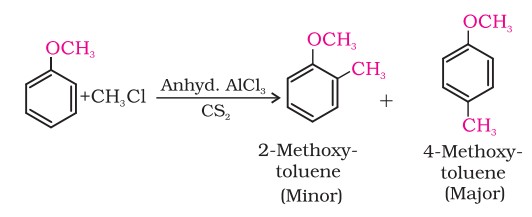
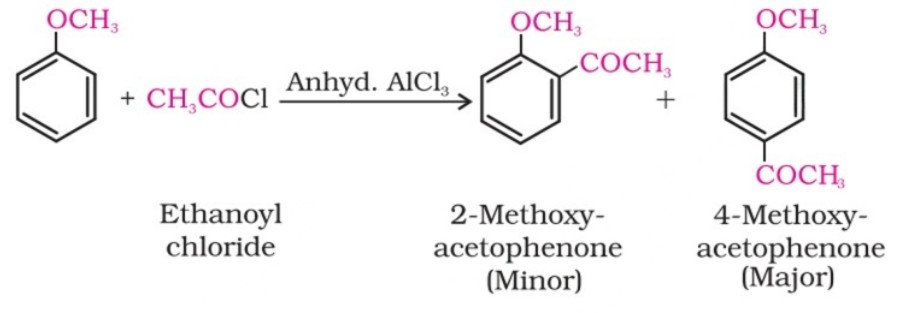
(ii) Friedel-Crafts reaction:
The alkyl and acyl groups are introduced at ortho and para positions by reaction with alkyl halide and acyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as a catalyst.


Chemical Reactions - Electrophilic Substitution (III)
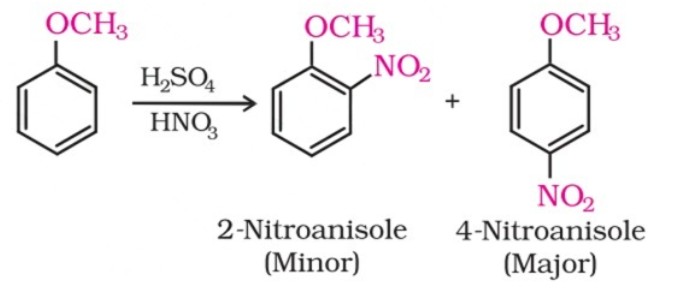
(iii) Nitration:
Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and para nitroanisole.


 beeTokens
beeTokens